Regulatory-Ready Reporting
Cross-Functional Integration
Study Design and Protocol Support
Data Review and Readiness
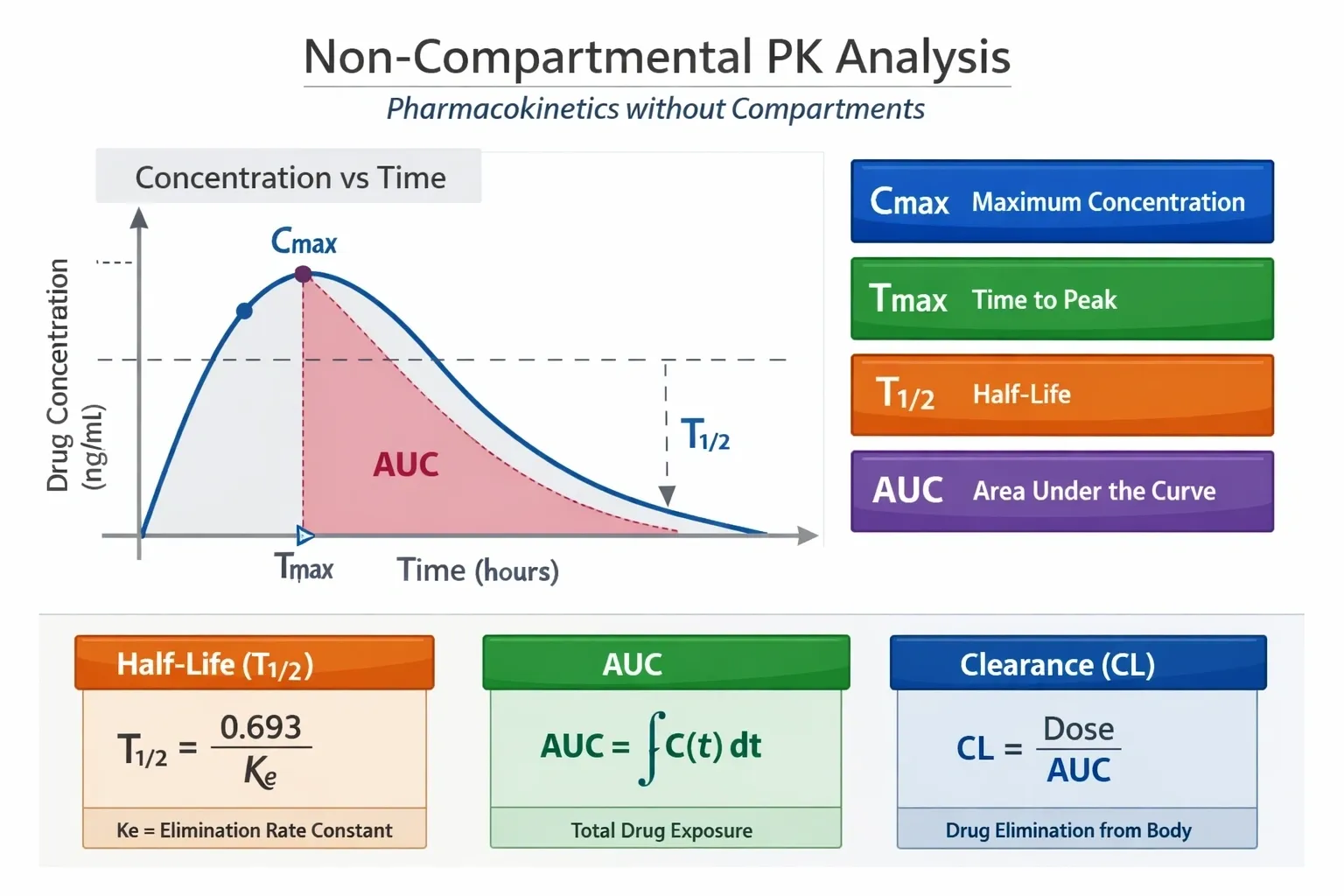
Noncompartmental PK Analysis
Interpretation and Decision Support
Our end-to-end noncompartmental PK support ensures that your clinical studies generate high-quality, interpretable PK data to support dose justification, labeling, and regulatory success across the drug development lifecycle.
Read PublicationsAt XP Pharma Consulting, we help you translate preclinical discovery data into actionable clinical strategies that support a successful IND submission (PK/PD analysis for IND submissions). Our team bridges nonclinical and clinical pharmacology through modeling, simulation, and regulatory alignment to ensure your first-in-human studies are scientifically sound and submission-ready.
Our expertise includes:
From preclinical data interpretation to IND dossier preparation, we guide you through each decision point with clear, quantitative insight.
In early clinical development, XP Pharma Consulting helps you design and execute studies that efficiently generate the data needed to support dose selection, safety characterization, and proof of concept. We integrate pharmacokinetic and pharmacodynamic insight with regulatory expectations to ensure every study fits seamlessly into your broader development strategy.
Our support includes:
From first-in-human to Phase 2a, we guide your early studies with precision and foresight — helping you move confidently toward pivotal trials.
At the pivotal stage of development, XP Pharma Consulting supports you in designing and interpreting complex studies that define the efficacy, safety, and exposure–response profile of your drug. We use advanced quantitative tools and regulatory experience to help you build a robust clinical pharmacology package for NDA/BLA submission.
Our team applies modeling and simulation to justify labeling doses, anticipate regulatory expectations, support pediatric strategies, and streamline development by reducing the need for additional late-phase studies.
Our services include:
Through precise modeling, data interpretation, and clear regulatory communication, we help you complete late-phase studies with confidence and prepare for a successful submission.
After completing early and late-phase studies, you’ve invested years of work and resources to bring your program to this critical point. XP Pharma Consulting partners with you to prepare the clinical pharmacology sections of your NDA/BLA submission, conduct integrated analyses, and provide clear, data-driven responses to regulatory agencies – regulatory strategy consulting for NDA/BLA.
Our team combines deep pharmacometric expertise with regulatory experience to ensure your submission meets FDA and EMA expectations, presenting a coherent, scientifically sound justification for dose selection, labeling, and risk assessment.
Our services include:
With XP Pharma Consulting, your submission benefits from scientific clarity, regulatory precision, and end-to-end support through approval.
©Copyright 2019 to 2026. All Rights Reserved