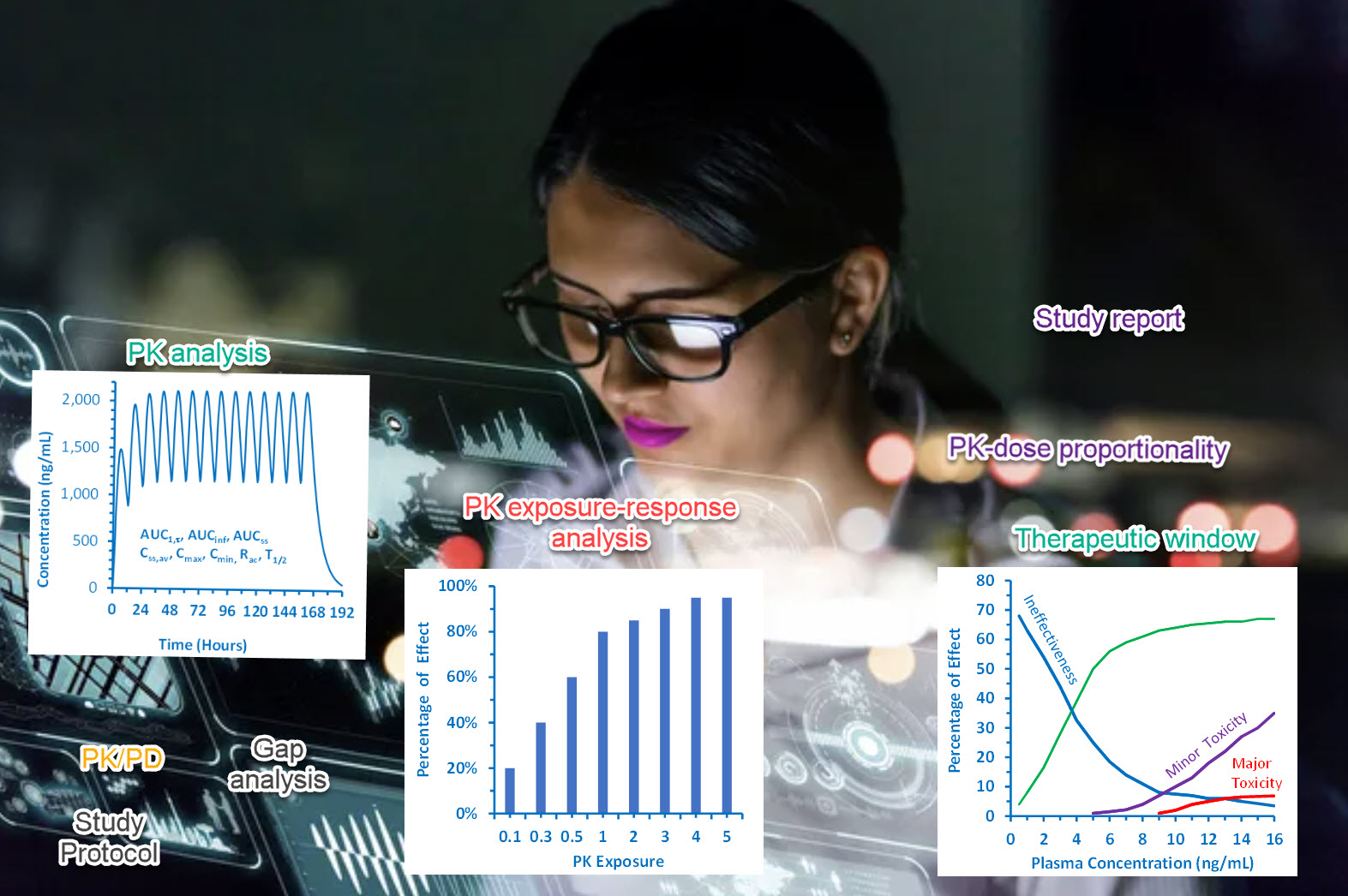
During FIH trial conduct, we performed non-compartment (NCA) PK analyses for each dose cohort on an ongoing basis. We found that the predicted and observed PK concentrations were in good agreement. The proposed dose range is adequate for RP2D selection.
The client was developing a molecule for the potential treatment of cancer with multiple mutations. For a New Drug Application (IND), the client needed an investigational plan for a first-in-human study (FIH). However, the selection of starting dose and dose range in the FIH study was a challenge: choosing a narrow dose range might not identify a recommended phase-2 dose (RP2D), whereas selecting a wider dose range would be an expensive and time-consuming addition to the study objectives for the RP2D.
XP Pharma proposed to choose starting dose and dose range selection using Modeling & Simulation technique in addition to traditional method with toxicology studies. We conducted a population pharmacokinetic (PK) modeling study across various species for allometric scaling to humans. PK exposures at various dose levels and schedules were obtained by simulation. Second, pharmacodynamic (PD) modeling was conducted to generate in vivo tumor-growth inhibition data. Linking the PK and PD models, the doses with the probability of achieving target concentrations for tumor growth inhibition were predicted, and a dose range was proposed for the FIH study. Our protocol was approved for the FIH study.
© Copyright 2024. All Right Reserved.