Dose Confirmation and Optimization
Population PK and Exposure–Response Analysis
Pivotal Phase 3 Trial Support
Special Populations and Bridging Strategies
Regulatory Strategy and Submission Support
Key Deliverables
At XP Pharma Consulting, we help you translate preclinical discovery data into actionable clinical strategies that support a successful IND submission (PK/PD analysis for IND submissions). Our team bridges nonclinical and clinical pharmacology through modeling, simulation, and regulatory alignment to ensure your first-in-human studies are scientifically sound and submission-ready.
Our expertise includes:
From preclinical data interpretation to IND dossier preparation, we guide you through each decision point with clear, quantitative insight.
In early clinical development, XP Pharma Consulting helps you design and execute studies that efficiently generate the data needed to support dose selection, safety characterization, and proof of concept. We integrate pharmacokinetic and pharmacodynamic insight with regulatory expectations to ensure every study fits seamlessly into your broader development strategy.
Our support includes:
From first-in-human to Phase 2a, we guide your early studies with precision and foresight — helping you move confidently toward pivotal trials.
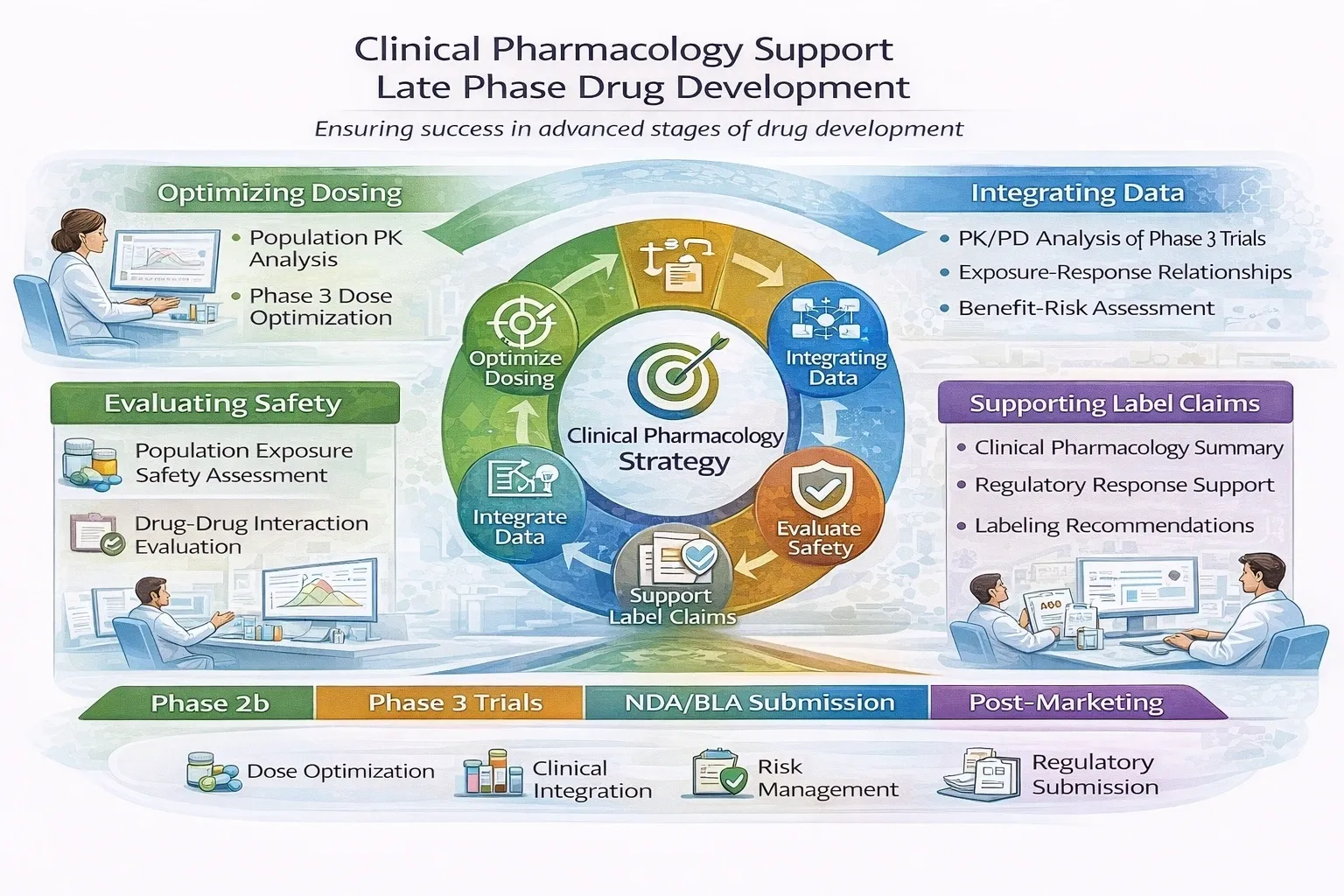
After completing early and late-phase studies, you’ve invested years of work and resources to bring your program to this critical point. XP Pharma Consulting partners with you to prepare the clinical pharmacology sections of your NDA/BLA submission, conduct integrated analyses, and provide clear, data-driven responses to regulatory agencies – regulatory strategy consulting for NDA/BLA.
Our team combines deep pharmacometric expertise with regulatory experience to ensure your submission meets FDA and EMA expectations, presenting a coherent, scientifically sound justification for dose selection, labeling, and risk assessment.
Our services include:
With XP Pharma Consulting, your submission benefits from scientific clarity, regulatory precision, and end-to-end support through approval.
©Copyright 2019 to 2026. All Rights Reserved