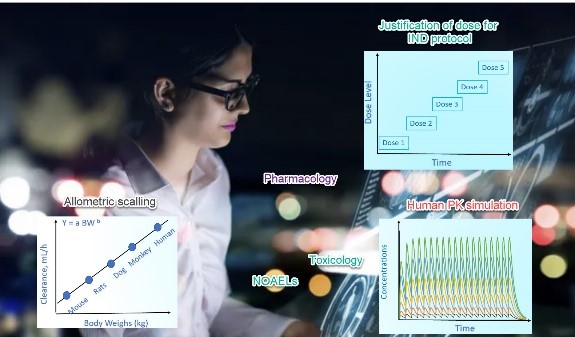
XP Pharma Consulting works with you to translate preclinical DMPK, toxicology and pharmacology data to clinical study design for IND opening study. We offer following services:
XP Pharma Consulting works with you to create plans to ensure that each study fits with your overall drug development program and facilitates efficient and timely data generation, analysis, and reporting. We offer the following services:
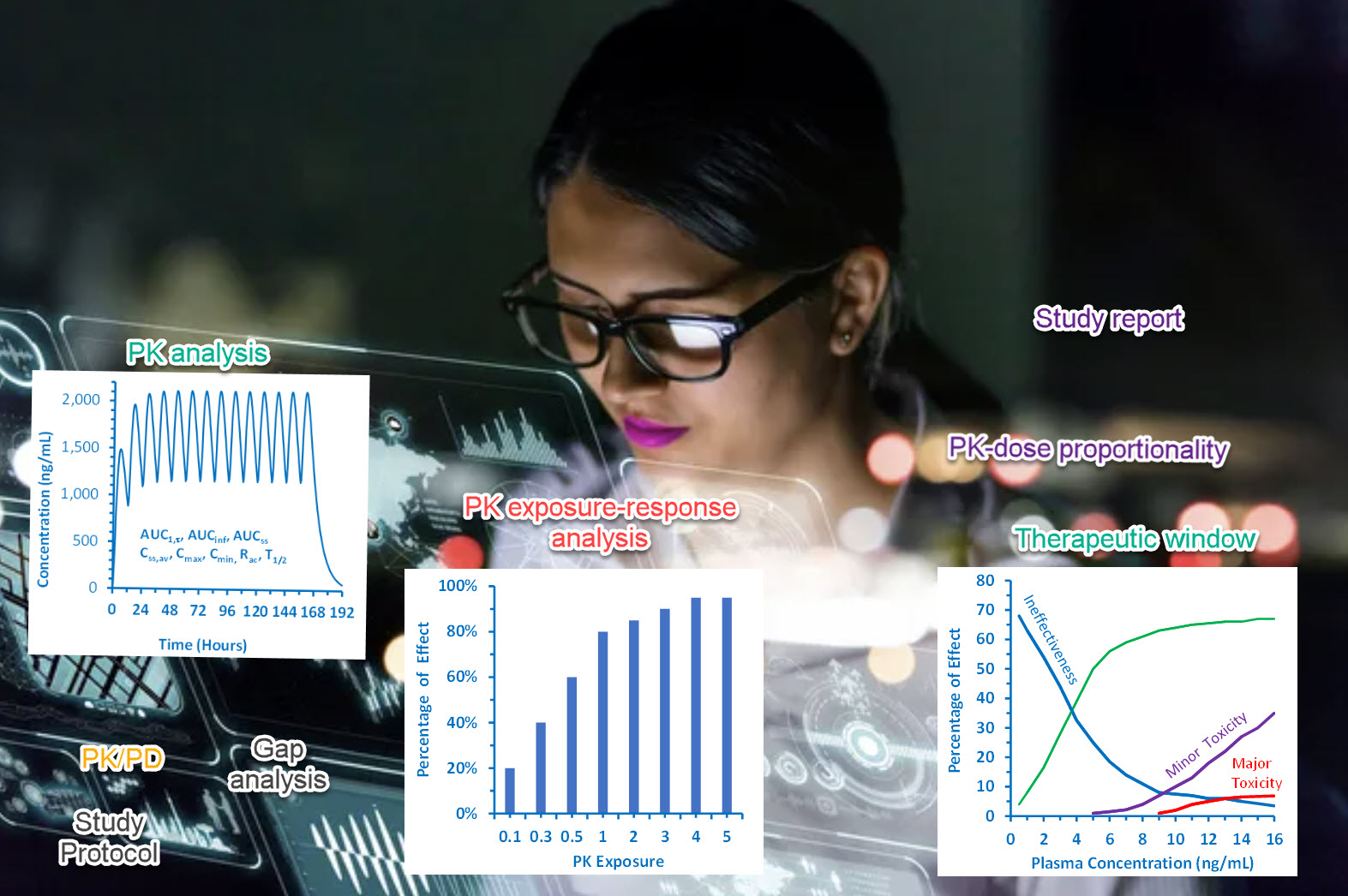
You have successfully completed several early phase studies. Now XP Pharma Consulting works with you utilizing quantitative tools and strategies that facilitate successful data interpretation and reporting. We help you design pivotal studies that will generate the PK/PD data required to secure a successful clinical pharmacology package for NDA/BLA filing. We employ innovative modeling and simulation techniques to justify the doses in the labeling, avoid certain late-phase studies, build a bridge strategy to pediatricpopulations, simulate clinical studies under different scenarios, and more:
Clinical pharmacology studies are necessary to characterize your drug’s pharmacokinetics (PK) and pharmacodynamics (PD) properties and for dosing instructions on the label. XP Pharma Consulting works with you to identify the necessary clinical pharmacology studies based on available nonclinical, DMPK, pharmacokinetic, pharmacodynamic, clinical data, and industry standards. We are confident in our recommendations to ensure you have what you need to achieve regulatory success for your drug development and registration. XP Pharma Consulting works has extensive experience in clinical pharmacology plan, study design, protocol authoring, PK/PD data analysis, study monitoring, and study reporting for various clinical pharmacology study types.

You have successfully completed early-phase and late-phase clinical studies after significant investment. XP Pharma Consulting works with you to prepare clinical pharmacology portions of NDA/BLA submission documents, conduct necessary pooled analysis, and response to regulatory questions. We offer following services:

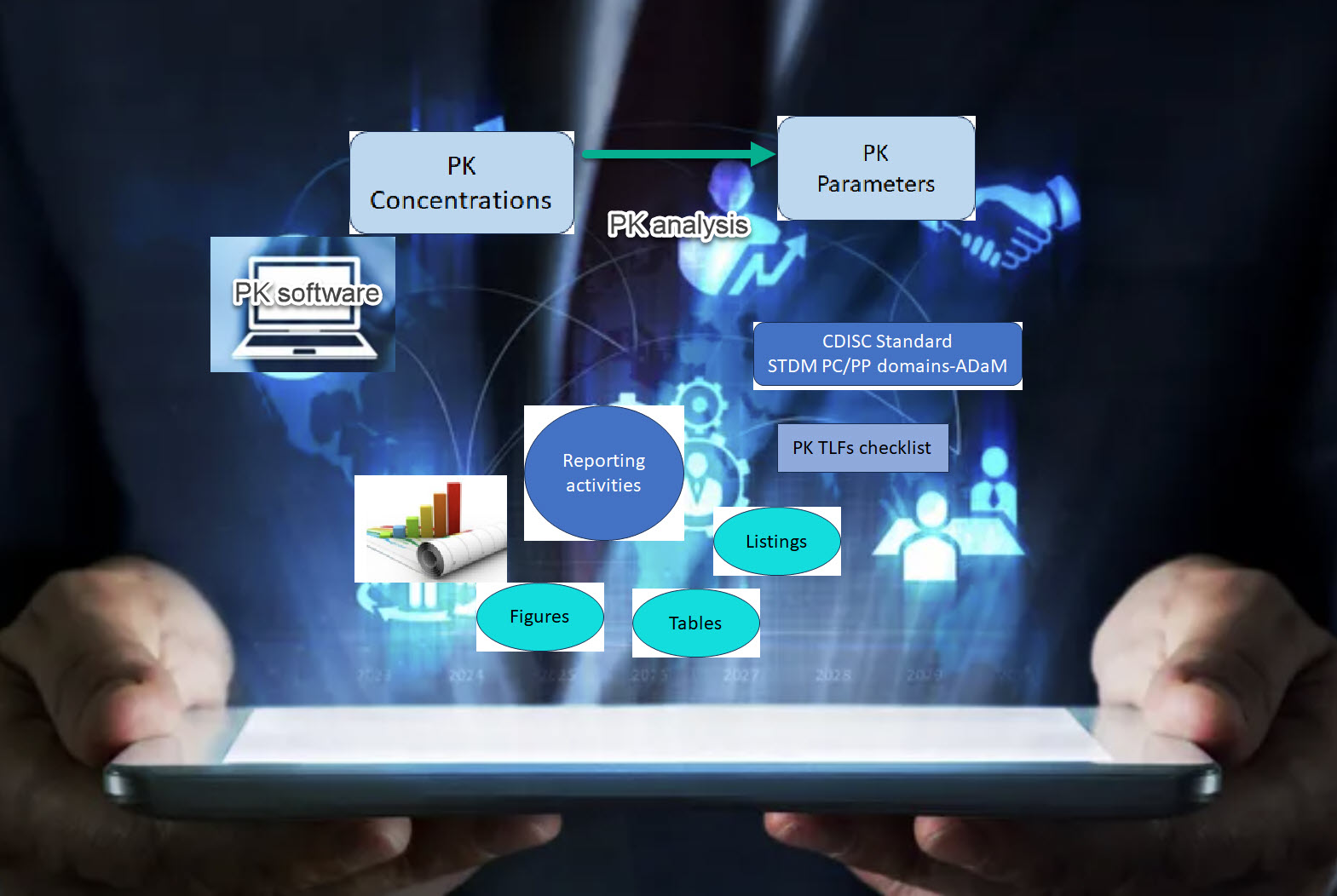
Noncompartmental pharmacokinetics (PK) analysis (NCA) is a standard method for calculating PK parameters. It is indispensable for characterizing PK within a single study and making time-critical dose selection decisions. XP Pharma Consulting works has extensive experience supporting end-to-end pharmacokinetics for clinical studies. We can help you with PK study design, data transfer, NCA analysis with validated software tools, and TLFs (Tables, Listings, and figures) to display results aligned with the PK analysis plan, results interpretation, and high-quality reporting. We ensure you get the most out of your data, setting your program up for success. We offer following NCA services:
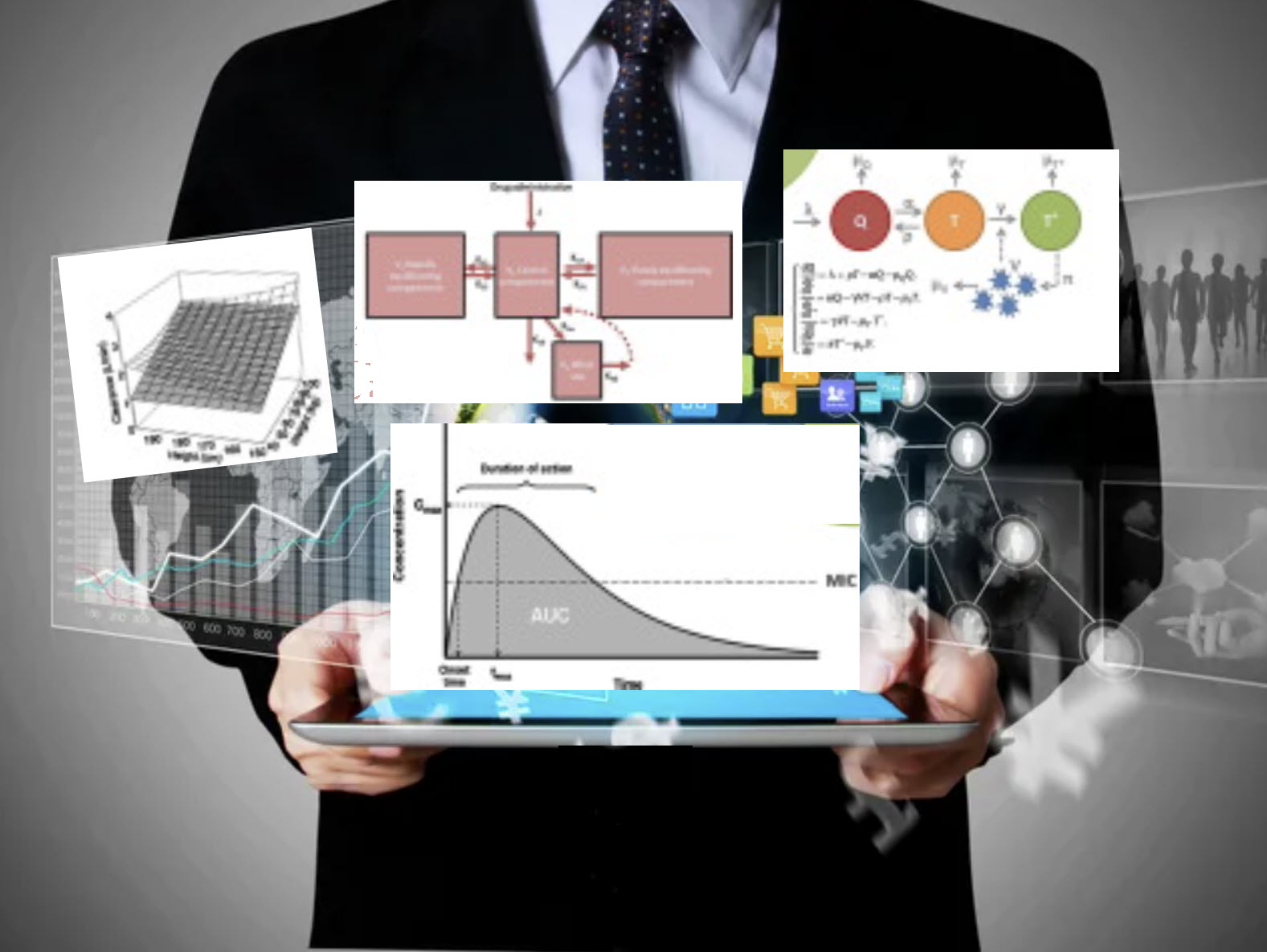
Regulatory agencies are responsible for approving all new drugs and ensuring that all available drugs on the market are effective and safe for human use. Understanding the safety and effectiveness of any drug depends, in large part, on pharmacokinetics (PK), pharmacodynamics (PD), PK/PD modeling and simulation (M&S). Because regulatory authorities increasingly emphasize M&S analyses and the wealth of information they provide, it is essential to consider M&S to advance your drug development program. XP Pharma Consulting works has expertise in M&S analysis and reporting. We can work with you to optimize dose and regimen, improve the probability of success, and ensure ideal labeling, prescribing, and an optimal patient experience. We offer the following services:
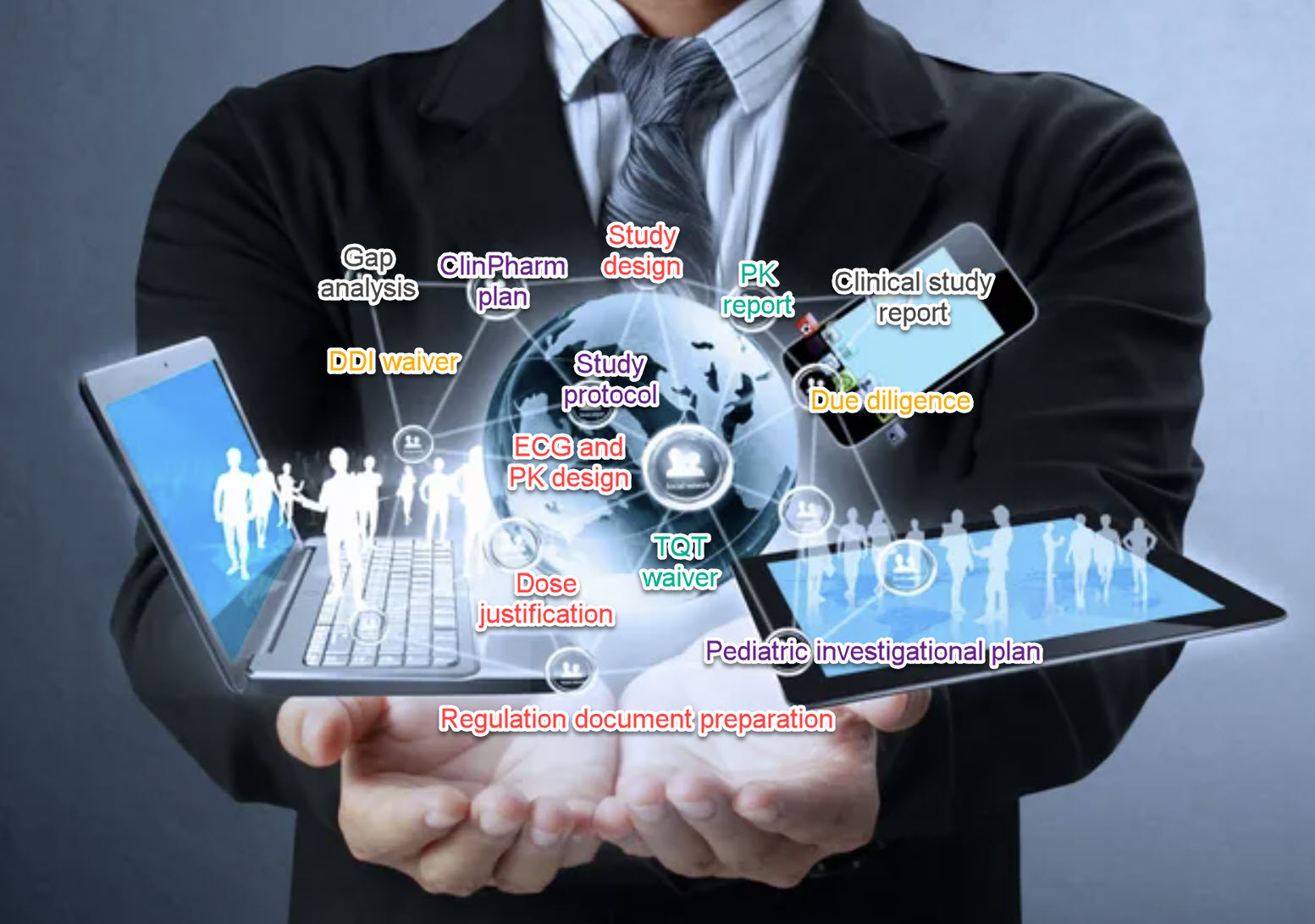
Clinical pharmacology presents many challenges along the development milestones to successful market approval. A streamlined clinical pharmacology strategy based on your drug’s class-specific properties is essential for efficiency and cost savings. XP Parma Consulting has intensive experience setting the strategy roadmap and helping you advance your drug development milestones. Our subject matter experts prepare regulatory documents specific to clinical pharmacology, pharmacometrics modeling, and simulation (M&S), and dose justification across the development spectrum from pre-IND to registration. We deliver high-quality written deliverables and submission-ready documents to facilitate drug development:
© Copyright 2025. All Right Reserved.